Some age-related decline may not be inevitable wear and tear. New research shows at least certain abilities are programmed to shut down.
Imagine suddenly losing your ability to smell certain foods; no longer sensing the aroma of fresh bread or ripe fruit. This is what happens to roundworms when they stop reproducing. They can still move and function normally, but they lose their ability to detect certain food odors that once led them to their meals. Their own genes have essentially betrayed them.
Researchers at Nagoya University have found the gene that causes this sensory decline. Published in Aging Cell, this is believed to be the first time scientists have identified a gene that actively shuts down food-odor detection in aging animals.
For decades scientists believed that aging happened mainly through accumulated damage. The idea that genes actively program specific decline rather than gradual deterioration is relatively new and controversial. Since similar genes exist in mammals, scientists can now study if they cause age-related decline in other animals, including humans.
What happens on day five?
Caenorhabditis elegans is a microscopic roundworm about one millimeter long that lives for only two to three weeks. These transparent worms reach adulthood in three days, reproduce for several days, and then age and die naturally. Their short lifespan allows scientists to study aging processes in weeks rather than years
“Day five is right after roundworms finish reproducing and have already passed their genes to the next generation. At this time these worms normally experience a sharp decline in their ability to sense diacetyl, an odor released by their bacterial food source,” Kentaro Noma, senior author and associate professor at Nagoya University Graduate School of Science, said.
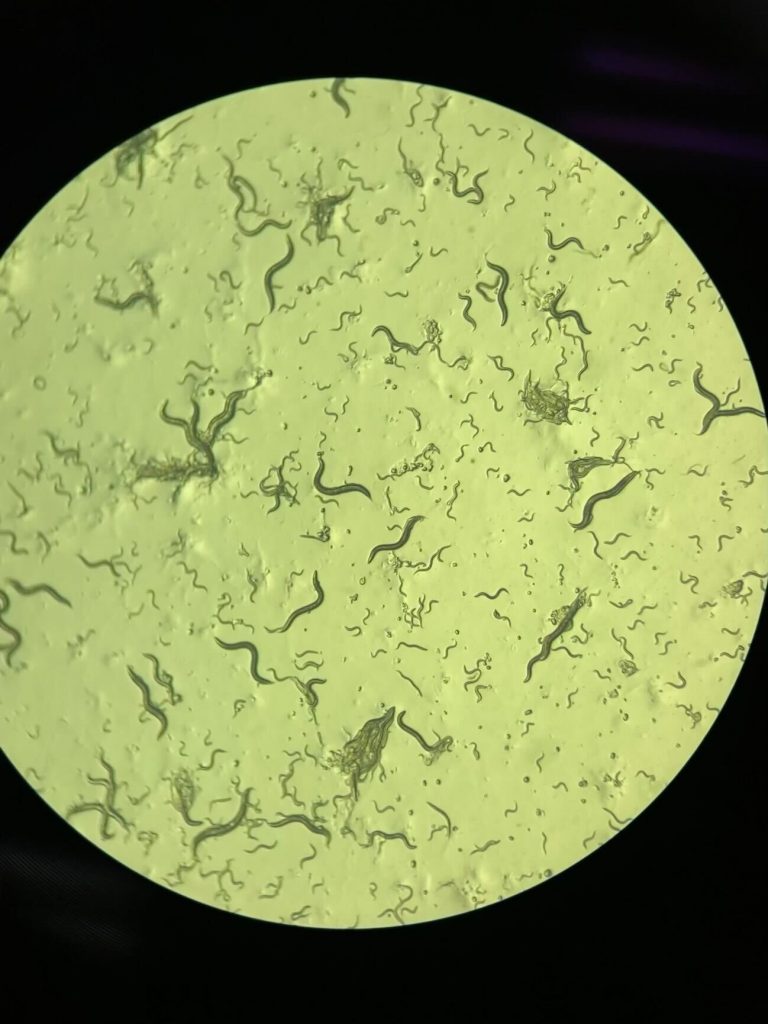
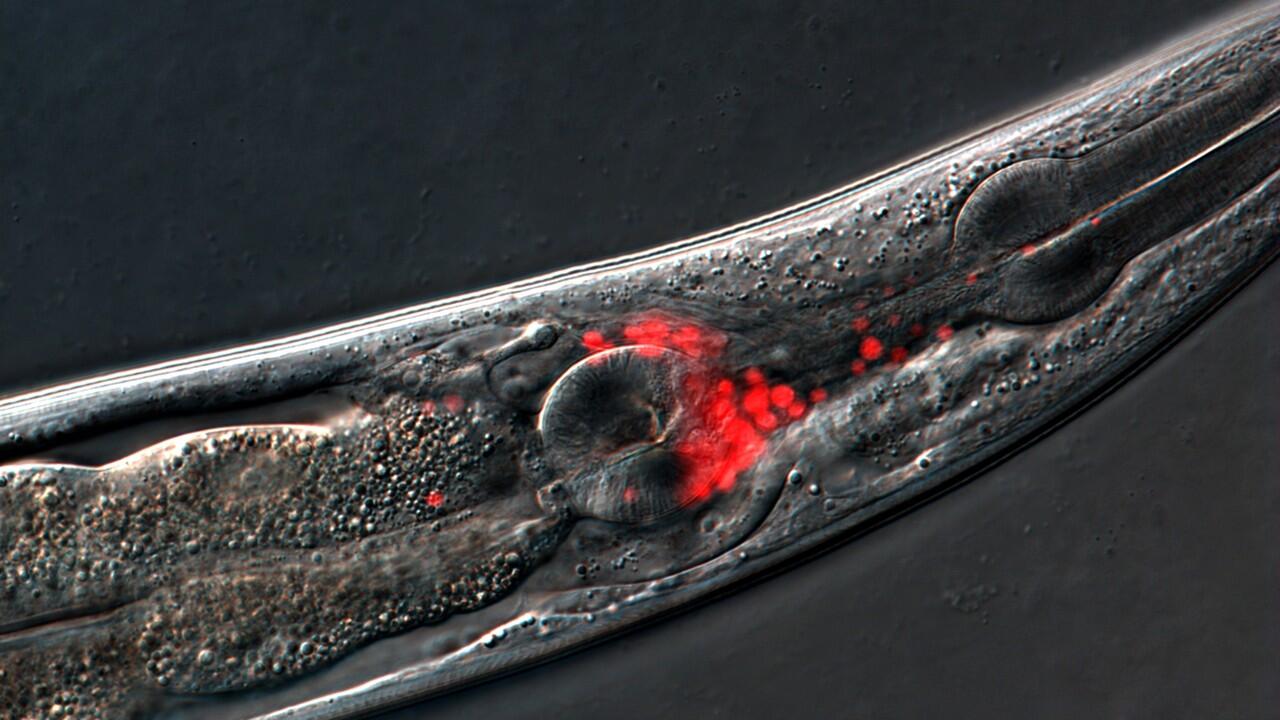
Tiny C. elegans worms show scientists how aging is programmed into their DNA. Scientists can observe their entire lifespan for a few weeks to study how genes control behavior at different ages. Credit: Noma Lab, Nagoya University
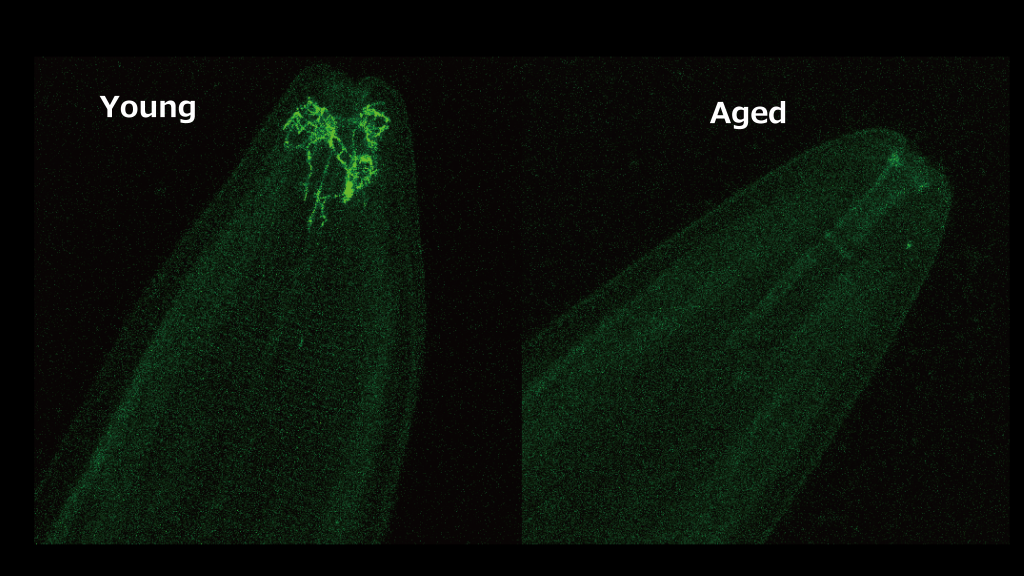
The researchers created worms with random genetic mutations. When they tested them on day five most had lost their ability to smell food, just like normal worms. However, some mutants kept this ability. These worms had a mutation in the nhr-76 gene which showed that this gene causes the decline in smell.
“nhr-76 produces a protein that switches off the genes responsible for detecting food odors. It works directly in the sensory neurons responsible for smell,” lead author and graduate student Rikuou Yokosawa explained. “The protein may receive a chemical signal to work, which suggests something in the aging body activates it.”
Why would an animal’s genes work against it?
Why does this harmful gene exist? There are two possibilities. Either natural selection simply cannot remove it because it acts after reproduction, or it actively evolved because the decline benefits the population by reducing competition for resources.
Evolution works by getting rid of genes that prevent organisms from reproducing. But once reproduction happens, any genes, even harmful ones, have already been passed to the next generation. This is why genes that cause decline after reproduction can persist; their negative effects appear too late for evolution to remove them.
However, the researchers propose a different explanation. The decline in food-seeking behavior after reproduction ends could benefit the population by reducing competition between older and younger animals for resources. If true, this active genetic program may have evolved to help offspring survive in a competitive environment.
While most aging research has focused on how damage accumulates over time, this study identifies a gene that actively causes a specific behavioral decline in roundworms. Whether similar programmed mechanisms contribute to human aging remains an open question.
“Similar genes (nuclear hormone receptors) exist in mammals, and there are hints they might play related roles in aging, but no clear parallel finding has been established in humans or other mammals yet. This is why this study is important; it provides a model that could guide future research in other species,” Professor Noma noted.
Paper Information:
Rikuou Yokosawa, Kentaro Noma. (2025) A Nuclear Hormone Receptor nhr-76 Induces Age-Dependent Chemotaxis Decline in C. elegans, Aging Cell, 24,12. https://doi.org/10.1111/acel.70277
Funding information:
This work was supported by JSPS (JP 21K06014), JST (JPMJFR214V), Daiko Foundation (Grant No.9286), and JST SPRING (JPMJSP2125).
Expert Contact:
Kentaro Noma
Graduate School of Science
Nagoya University
Email: noma.kentaro.f1@f.mail.nagoya-u.ac.jp
Media contact:
Merle Naidoo
International Communications Office
Nagoya University
Email: icomm_research@t.mail.nagoya-u.ac.jp
Top image:
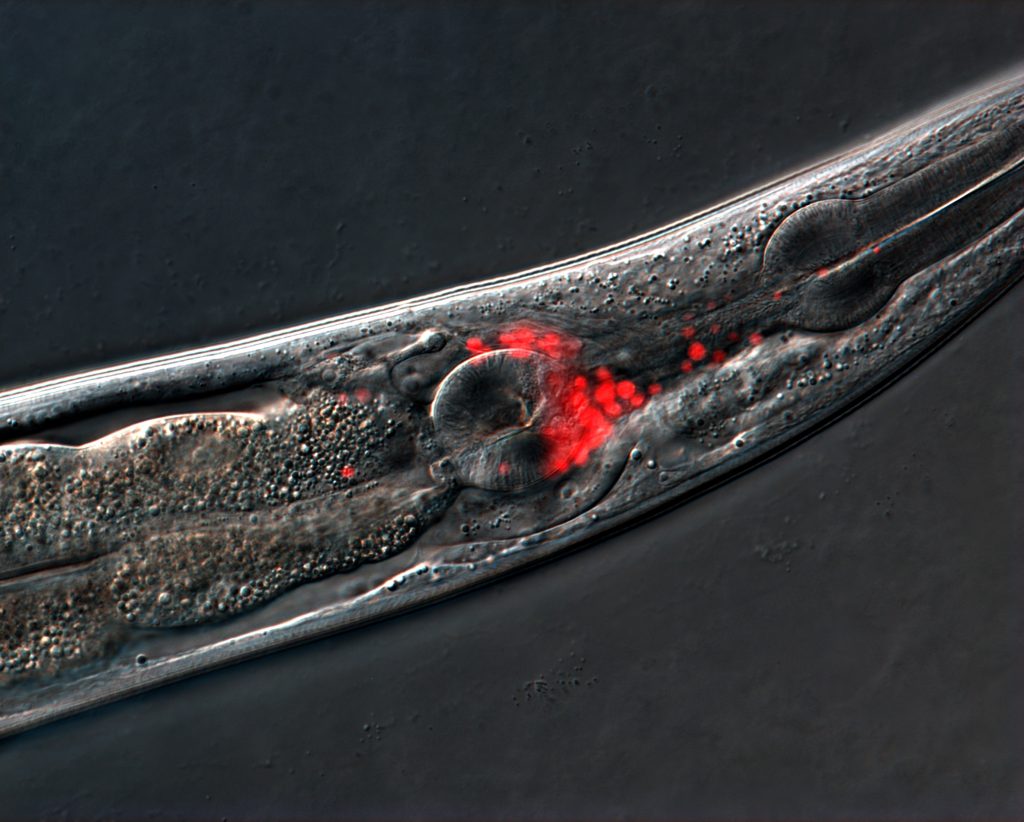
C. elegans roundworms engineered with fluorescent proteins to visualize neurons. Credit: Noma lab, Nagoya University